
网页NDC 51662-1390-1 MAGNESIUM SULFATE INJECTION, USP 50% 1gram per 2mL (500mg per mL) 2mL VIAL HF Acquisition Co LLC, DBA HealthFirst Mukilteo, WA 98275 Also supplied in the following manufacture supplied dosage forms
网页2010年1月1日 · But there is no standardized approach to stability evaluation.” Reagents subject to stability issues include individual reagents, kits, associated calibrators and
网页We also assayed pentobarbital content over time in preparations of various ages up to 6 years old. Results: We determined that the drug degraded at a maximum of 0.5% per year in our preparation (alkaline water/propylene glycol/ethanol) when stored in the dark at room temperature. A yellow discoloration developed after about 2 years, which we
网页2011年2月5日 · This video shows the simple 3 step process of using the Thomson Filter Vial. Thomson's filtration products are engineered and optimized to increase the effi This video shows the simple 3
网页2010年2月1日 · Purpose: The stability of cetuximab and panitumumab in glass vials and polyvinyl chloride (PVC) bags stored at 4 degrees C for up to 14 days was studied.
网页2010年1月1日 · But there is no standardized approach to stability evaluation.” Reagents subject to stability issues include individual reagents, kits, associated calibrators and controls, and even sample diluents. Pierson-Perry explains that
网页Annex I: General safety and performance requirements. Annex II: Technical documentation. Annex III: Technical documentation on post-market surveillance. Annex IV: Eu declaration of conformity. Annex V: CE Marking of conformity. Annex VI: Information to be submitted upon the registration of devices and economic operators.
网页2022年6月3日 · Filter stability will be discussed further in § 8.4 after poles and zeros have been introduced. Suffice it to say for now that, for stability, the feedback coefficients must be restricted so that the feedback gain is less than 1 at every frequency. (We'll learn in §
网页stability studies, as some oil formulations are susceptible to degradation before 180 days. Additionally, several commenters noted that oil formulations may not always be clinically appropriate. BUDs in USP <797> New factors for
网页2022年12月20日 · Chapter written by Michel BARRET. Learn more about Chapter 10: Filter Stability on GlobalSpec. Providing a preliminary step for the analog-to-digital filter
网页After initial entry into the vial, the remaining contents must be used within 48 hours. Infusion Systems for Intravenous Administration The following infusion systems have been tested and found satisfactory: unit-dose glass containers;
网页Filter stability will be discussed further in § 8.4 after poles and zeros have been introduced. Suffice it to say for now that, for stability, the feedback coefficients must be restricted so
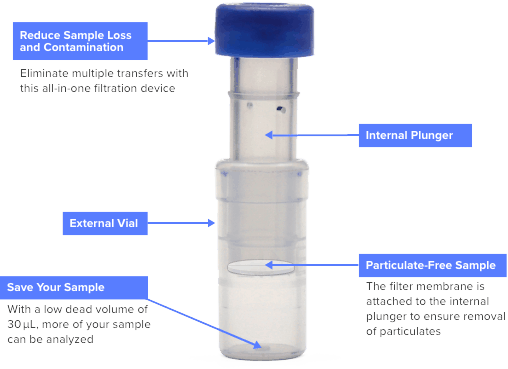
网页low volume, viscous, particulate-laden or high volatility organic solvent Thomson has a Filter Vial to fit your needs. Key Features. Same Size as a standard HPLC Vial and will fit easily into any machine or tray available for standard HPLC vials. Available in PTFE, PVDF, PES and Nylon membranes. Pore sizes of either 0.2μm or 0.45μm.
网页include a filter. Do not infuse mannitol solution if crystals are present. 7. Do not administer unless solution is clear and container is undamaged. Discard unused portion. Do not administer 25% mannitol if the fliptop vial seal is not