
2017/05/15 · Leachables: Organic and inorganic chemical species that can be released from the surfaces of components used in the manufacture and storage of drug products under conditions of normal use. Extractables represent the worst-case scenario regarding release of mobile chemical species from manufacturing and packaging components during forced
Viral Quantification Adeno-Associated Virus Vector Genome Titer Assay ddPCR Results We compared an AAV genome titer assay based on ddPCR with a standard and an optimized
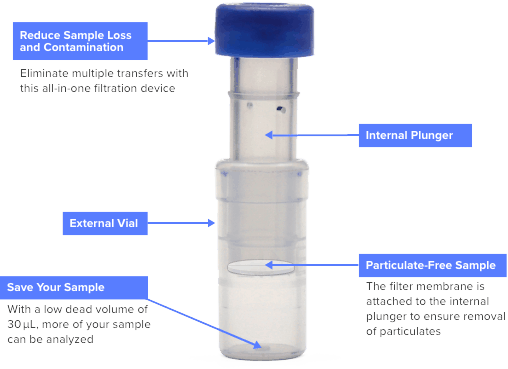
Captiva filter vials reduce the steps in your gas chromatography (GC) or high-performance liquid chromatography (HPLC) workflow. Just fill, cover, and plunge! Filter vials are a fast,
2018/05/22 · Limit of Detection (LOD) The limit of detection LOD (or detection limit, DL) is the lowest possible concentration at which the method can detect (but not quantify!) the analyte within the matrix with certain degree of confidence. It is also defined as the lowest concentration that can be separated from a background noise with some reliability.
This study exploits the virulent bacteriophages phi 6 (dsRNA) and MS2 (ssRNA) as surrogates for airborne RNA viruses. Two different filter types, polytetrafluoroethylene (PTFE) and
GVS 社製Filter Vial(フィルター付ミニバイアル) SEPARA 近年、お客様から大変多くのご要望がありました 『Filter Vial:フィルターとPP製のバイアルを一体化したフィルター内蔵バイアル』を発売致しました。 シリンジレスのオールインワン構造により従来の常識を覆すスピーディーなサンプルろ過に
2021/02/04 · The stationary and mobile phases were contained within an Aijiren Series II LC System (Aijiren, Waldbronn, Germany) consisting of an automated, thermally-controlled vial sampler
Quantification with qPCR showed 37–62% difference between determined average vector quantity of samples, when using DNase and proteinase K treatment in comparison to DNase only treatment (). For the first set of samples (1–5 in Table 3 ), the determined qPCR titer based on the pre-defined concentration of standard curve material, was on average 64× higher than titer
Separa® Filter Vial. The device consists of an internal insert with a membrane chamber and precut septa cap and an external vial. A sample ready to use after filtration. Pre-slitted cap ensures easy and clean sample transfer. Replace syringe, syringe filter, glass vial, and cap, reducing waste. Increase sample integrity with all-in vial and
2017/09/01 · Contamination with foreign particulate matter continues to be a leading cause of parenteral drug recalls, despite extensive control and inspection during manufacturing. Glass is a significant source of particulate matter contamination; however, the mechanism, source, and quantification have not been extensively analyzed. Quantification of particulate matter
廃棄物の量を減らすことができます シリンジフィルターを用いた分析前のサンプル濾過では、シリンジ、シリンジフィルター、ガラスバイアルの3つが必要でした。その保管に場所を取ったり廃棄物が増えてしまう懸念がございましが、フィルターバイアルを使用すればその課題を解消す
Made of Type I Borosilicate Glass, SCHOTT Pharma´s vials offer high chemical resistance for the secure storage of liquid drug formulations. Their accurate dimensions and superior cosmetic quality ensure an efficient fill-and-finish process and secure container closure integrity. In addition, special features such as improved inner surfaces
Presented in this unit is a method for total HIV DNA quantification of various HIV genome targets that utilizes the next-generation PCR platform, digital PCR. The abundance of various HIV gene targets reflects the overall proviral composition. In this protocol, total genomic DNA is isolated from patient-derived cells and then used as a template