
warm the vial using a dry heat cabinet, or warm water bath and vigorously shake the vial. Explosions of glass ampuls has occurred when mannitol crystals were resolubilized using a microwave.(14) Supersaturated mannitol solutions in PVC bags may cause a heavy white flocculant pre-cipitate to develop within minutes.
if a high risk sterile eye drop is compounded and a 14 day room temperature BUD is given, enough extra of the eye drop must be compounded to send for sterility testing. Single Dose Containers (SDC) and Multiple Dose Containers (MDC) SDC – vial, bag, bottle, syringe Outside ISO 5 environment – one hour from time of puncture or opening
Aseptic Technique for Vials To prevent vacuum formation, inject air into the vial equal to the volume to be withdrawn. When reconstituting a powdered drug, withdraw a volume of air equal to the amount of the diluent to be added. This will prevent positive pressure from developing inside the vial.
12 hour BUD room temperature or 24 hour BUD refrigerated CAI and CACI in compounding suite may have longer BUDs Pharmaceutical Compounders (PC) in SCA may only be used for Category 1 CSPs 12 hour BUD room temperature or 24 hour BUD refrigerated PC in ISO 8 room may have longer BUDs Anteroom not required
Sep 3, 2017 · Use the following data: Multiples of 80mg/ 125 ml fluid - 6hr stability [ concentration: 0.64 mg/mL] 80mg/100ml - 4hr stability [ concentration: 0.8 mg/mL] Min dilution - 80 mg/75 ml - 2 hr stability [ concentration: 1.07 mg/mL] Example: 375mg/500mL [Conc = 0.75 mg/mL] Estimated stability ~ 4 hrs (rounding concentration to 0.8 mg/mL)
Sep 3, 2017 · Dilute 2 mg/ml (10 ml vials) or 4 mg/ml (10 ml) vials 1:1 with D5W or NS. May add to viaflex bag. Alternatively: Glass bottle: 40 mg/250 ml (250ml D5W). Infusion Rate: As directed. (See comments). Primary: Dilution for 2 mg/ml - 10 ml vials. == Final Concentration 1 mg/ml == 20 mg/20 ml (10 ml diluent + 10ml of drug) Total volume =20ml.
A multi-dose vial is a vial of liquid medication intended for parenteral administration (injection or infusion) that contains more than one dose of medication. Multi-dose vials are labeled as such by the manufacturer and typically contain an antimicrobial preservative to help prevent the growth of bacteria.
Beyond-use date (BUD) and storage requirements Quality control procedures (e.g., pH, filter integrity, and visual inspection) Sterilization method, if applicable (e.g., filter, steam, or dry heat) Any other information needed to describe the operation and ensure its consistent repeata - bility (e.g., adjusting pH, tonicity and/or temperature)
CSP’s or CNSP’s BUD identifies the time by which the preparation – once mixed – must be used before it is at risk for physical or chemical degradation, microbial contamination and proliferation, and impact on the integrity of the container-closure system. In other words, the BUD serves to alert healthcare workers to the time/
Instructions. Separate out about 2 grams of ganja. Break that bud into four pieces (roughly a ½ gram each). Heat the flat iron to between 300℉ and 330℉. Too hot and you’ll burn off a large portion of the chemicals you’re trying to extract. Too cool and you won’t get enough of the chemicals you’re trying to extract.
environment, and if the vial is one of three or fewer sterile ingredients, then this qualifies as either a Low-risk Level CSP or a Low-Risk Level CSPs with 12-Hour or Less BUD, depending on whether or not the primary engineering control, PEC or ISO Class 5 source is located in an ISO Class 7 buffer area.
Apr 21, 2018 · How supplied Reference Reference (s) National Institutes of Health, U.S. National Library of Medicine, DailyMed Database. Provides access to the latest drug monographs submitted to the Food and Drug Administration (FDA). Please review the latest applicable package insert for additional information and possible updates.
medication vial septum for multiple uses. This provides a direct route for microorganisms to enter the vial and contaminate the fluid.” Therefore, if a spiking or other device is used to puncture the MDV, the vial should remain in the ISO Class 5 environment for subsequent uses. See http://www.cdc.gov/injec Does not regulate practice.
(D5W). Contents of vials may be pooled under aseptic conditions into sterile infusion bags. Use within 8 hours after pooling. Warm to room temperature prior to infusion. If dilution is required, may be diluted with 5% dextrose in
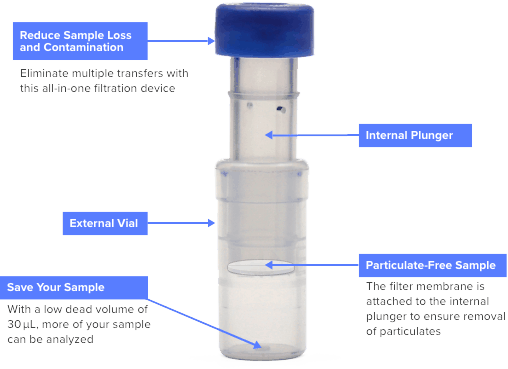
Filter Needle II Removable 5 micron filter with attached 19 G x 1 in. thinwall needle 415002 0.2 micron PF2000 Designed for bacteria retentive filtration of medication; USP <797> compliant for sterilizing pharmaceutical fluids. Filter Needles with 5 micron filter in female luer lock connector.