
Manufactured via a 2-, 3-, or 4-step certification process, we ensure that Aijiren Technology Certified Vials meet and maintain specified vial dimensions, levels of cleanliness and adsorption properties from batch to batch. Find the right vial for your application and autosampler below or use our Vial Selector to find your perfect match. Shop Now
Jul 20, 2022 · Each filter paper was placed in a glass vial, and 2 mL of ultrapure water was used to rinse filter papers under sonication for 2 min. The liquid was transferred to 50 mL of ZnCl 2 solution (900 g/L, filtered before use), and vial was rinsed with ZnCl 2 solution several times to ensure that no MPs were lost.
There have been several revisions to the analytical procedures outlined in the bacterial endotoxin test since it was first issued in 1980. These changes have enabled the LAL method to be more
A crucial undertaking when releasing pharmaceutical products for the market, is to determine the purity of the final product, necessitating the need to determine its impurity profile. Traditionally, this was concerned only with those impurities arising from the manufacture and degradation of the pharmaceutical product.
Jun 8, 2018 · The sample was automatically delivered to a tube containing a filter, to which reagents were added, mixed, and then filtered. Precisely, 100 μL of acetonitrile was added to a polytetrafluoroethylene (PTFE) filter vial (0.45 μm pore size) previously conditioned with 20 μL methanol.
Quantification of particulate matter generation with lab simulations suggests that glass-to-glass contact on the filling line produces large quantities of glass particles of various sizes. A new strengthened glass vial with a low coefficient of friction surface is proposed to address this root cause of glass particle generation.
Sep 10, 2021 · A small quantity of PE microspheres (90–106 μm) were placed on a black PC filter, the filter was dyed with 400 μl of a working solution, air-dried for 5 min, and a preparat was fabricated and
Mar 5, 2022 · Figure 6.3. 1: Membrane filters can be used to remove cells or viruses from a solution. (a) This scanning electron micrograph shows rod-shaped bacterial cells captured on the surface of a membrane filter. Note differences in the comparative size of the membrane pores and bacteria. Viruses will pass through this filter.
Nov 9, 2020 · This new technology has higher sensitivity, is more robust than conventional kinetic testing, and provides quantitative and accurate endotoxin results. The endotoxin test method was developed with strong focus on endotoxin recovery from all representative elastomer components.
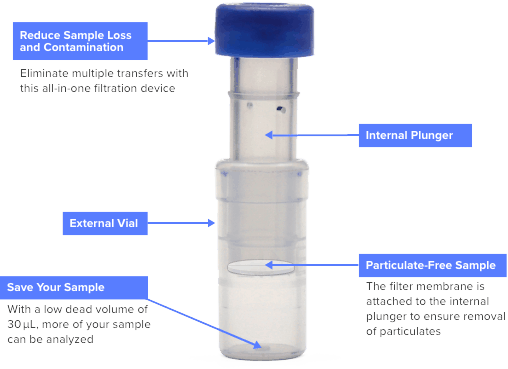
Verex Filter Vials combines syringe filter and vial technology, eliminating the need for separate syringes, syringe filters, vials, and cap/septa, allowing you to reduce lab waste and simplify your workflow. The Most Innovative 4-in-1 Technology Table of Contents 3 Verex Filter Vial Specifications • •Dimensions: 12 x 32 mm
Mar 16, 2017 · In addition to visual quantification, stock solution was prepared at 1 mg mL −1 in acetone and filtered using a 0.22 μm PTFE syringe filter into a clean glass screw-top vial and used for ...
Sample Preparation by Filtration. Filtration is a separation technique used to concentrate or purify substances based on their physical or chemical properties. It is a simple and routine method used in many laboratories to remove insoluble particles from solutions and to prepare samples for analysis. Filtration is used to reduce sample
Filter Validation Protocol. The Aijiren 850-DS Dissolution Sampling Station is optionally equipped with a fi ltering option that greatly improves dissolution sample processing through the use of innovatively designed fi lter plates. The WhatmanTM850-DS 8-Channel Filter Plates from GE Healthcare, are consistent with fi ltration membranes and
Our flow cytometer alignment and cell sorting beads are designed to help ensure your flow cytometer is performing at its peak, your experimental design is robust, and the data you collect and analyze are accurate. In addition, our size calibration and size reference kits serve as reliable size references for flow cytometer users. On demand webinar
3. Aliquot 1 mL into a new vial. Centrifuge at 5,000 rpm for five minutes. 4. Transfer 50 μL of supernatant to a new vial. Add 950 μL of methanol. Mix briefly. (20 fold dilution for a total of 2,000 fold) 5. Filter with 4 mm, 0.45 μm regenerated cellulose (RC) syringe filters (p/n 5190-5107). Sample preparation for oils and concentrates 1.