
Thawed vials can be handled in room light conditions. Thawed vials can be handled in room light conditions. Thawed vials can be handled in room light conditions. Gently invert the vial 10 times to mix before and after adding the diluent, 1.8 mL of 0.9% sodium chloride. Do NOT shake the vial. Gently invert the vial 10 times to mix before and
Filter Needle: A filter needle has a 5 micron filter at the base of a syringe needle. The filter creates a one-way flow when withdrawing or injecting fluid into or from the syringe. The filter needle can be used either to withdraw or to inject but never for both; it should only be pulled or pushed in one direction.
If the amount of vaccine remaining in the vial cannot provide a full dose, discard the vial and contents. Do not pool excess vaccine from multiple vials. Discard vial 12 hours after first puncture, even if vaccine remains in the vial. Record date and time of the first use on the vial label; Administer Moderna COVID-19 vaccines intramuscularly
Sep 27, 2020 · CDC publishes and routinely updates a comprehensive Vaccine Storage and Handling Toolkit covering topics such as vaccine storage units, temperature monitoring devices, and inventory management, vaccine transport and emergency vaccine storage and handling. The toolkit also contains troubleshooting guides to assist with vaccine storage unit
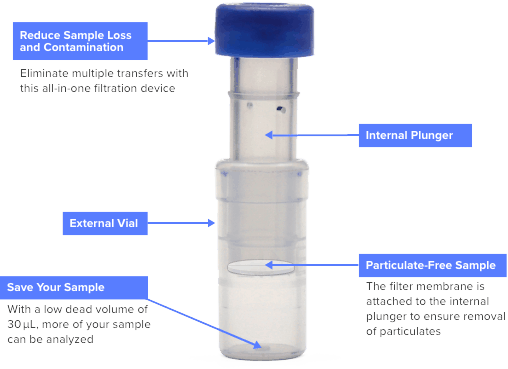
Captiva filter vials remove particulates from your sample and are ideal for simple mechanical filtration. Filtering samples before analysis can extend column lifetime, decrease instrument downtime, and improve sample integrity. Captiva filter vials reduce the steps in your gas chromatography (GC) or high-performance liquid chromatography (HPLC) workflow.
Multidose vials should be accessed on a surface that is clean and where no dirty, used or potentially contaminated equipment is placed or stored. Scrub the access diaphragm of vials using friction and 70% alcohol. Allow to dry before inserting a new needle and new syringe into the vial.
• The vaccine vial monitor (VVM) that is attached to the vaccine vial can serve as a visual trigger to assist a health worker in properly applying the multi-dose vial policy, especially in knowing when the reconstituted product must be discarded.
Apr 19, 2018 · Filtration methods include membrane technology (microfiltration operated in normal-flow filtration (NFF) mode, tangential-flow filtration (TFF), or depth filters operated as NFF. Here we provide a comprehensive overview of different filtration technologies and their application in viral vaccine clarification.
Vaccine Administration: Preparation and Timely Disposal. Vaccines should be drawn up in a designated clean medication area that is not adjacent to areas where potentially contaminated items are placed. Multi-dose vials to be used for more than one patient should not be kept or accessed in the immediate patient treatment area.
earliest date. Do NOT use vaccine if the expiration date or beyond-use time has passed. Administration COVID-19 vaccine may be administered at the clinical visit as other vaccines. Do not "pool vaccine" from more than 1 vial to obtain a dose. If a full dose cannot be withdrawn, discard the vial and any remaining vaccine.
Prepare the Vaccine (s) Proper preparation is critical for maintaining the integrity of the vaccine during transfer from the vial to the syringe. Always use aseptic technique and follow infection prevention guidelines when preparing vaccines. Aseptic technique refers to the manner of handling, preparing, and storing medications and injection equipment/supplies (e.g., syringes, needles) to prevent microbial contamination and infection.
Jul 29, 2020 · The glass used to make vials for vaccines and other medications is scarce, and with the looming need to store and distribute COVID-19 shots on a massive scale, Honeywell aims to step in with a well
Apr 30, 2021 · Pfizer says it will offer smaller shipment sizes at the end of May in order to give vaccine sites more flexibility. Its current shipment size is a 195-vial pack that contains 1,170 doses. The
Cognex Deep Learning combined with High Dynamic Range (HDR+) technology is an ideal solution for particulate matter inspection. Cognex Deep Learning is trained on all types of particles appearing in vials and ampoules—the various shades and sizes, whether or not mixed with bubbles, and their appearance through the range of reflections and refractions of vial and ampoule glass.
Feb 2, 2021 · Covid-19 vaccines that use mRNA, such as those made by Pfizer/BioNTech and Moderna, require storage at around -80C. Valor glass can withstand temperatures from between -180C and 400C. Resistance